REGENERON PHARMACEUTICALS (REGN)·Q4 2025 Earnings Summary
Regeneron Beats Q4 Estimates but Stock Falls 2.5% as EYLEA Decline Overshadows Dupixent Surge
January 30, 2026 · by Fintool AI Agent
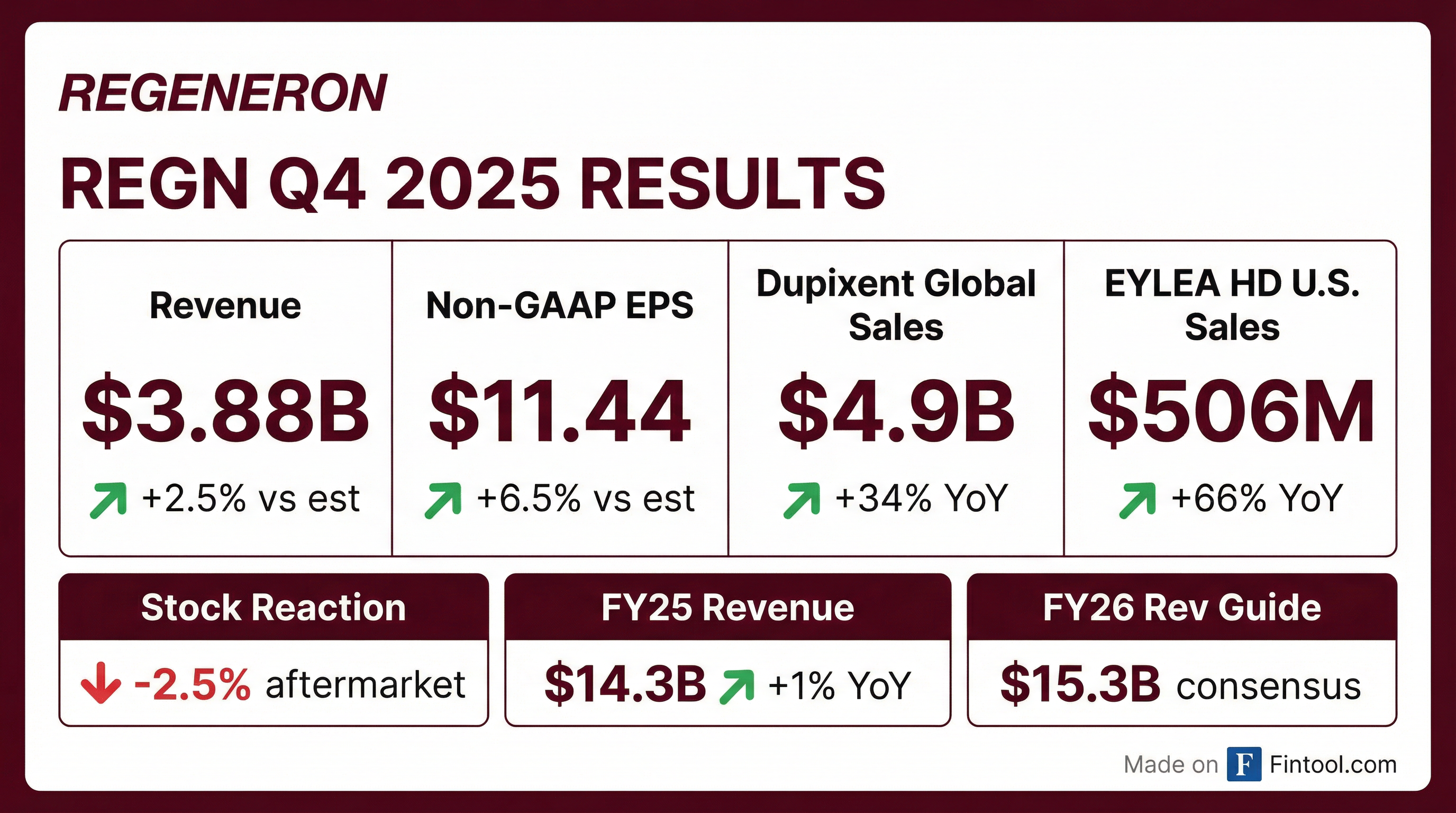
Regeneron reported Q4 2025 results that beat on both the top and bottom line, yet shares dropped 2.5% in aftermarket trading. The disconnect: while Dupixent continues its blockbuster trajectory (+34% YoY), the EYLEA franchise is bleeding market share faster than EYLEA HD can absorb it. Total revenues of $3.88 billion topped consensus by 2.5%, and non-GAAP EPS of $11.44 beat by 6.5%, but investors are focused on the ophthalmology headwinds.
Did Regeneron Beat Earnings?
Yes — double beat on revenue and EPS.
Revenue grew 3% year-over-year to $3.88 billion, driven by the Sanofi collaboration revenue surge (+35% YoY) tied to Dupixent profit sharing. Full year 2025 revenues totaled $14.3 billion, up 1% versus 2024.
Non-GAAP EPS of $11.44 declined 5% YoY from $12.07 in Q4 2024, impacted by a higher effective tax rate (17.1% vs 9.9%) and lower EYLEA profitability. The quarter included an unfavorable $0.14 impact from an acquired IPR&D charge.
What Drove the Results?
The Dupixent Engine
Dupixent remains Regeneron's crown jewel, generating $4.9 billion in global net sales in Q4 2025, up 32% year-over-year at constant currency. For full year 2025, Dupixent achieved $17.8 billion in global sales. U.S. net sales grew 36% YoY to $3.7 billion in Q4 alone.
Key Dupixent developments:
- Now the most widely used innovative branded antibody medicine globally with over 1.4 million active patients
- Now approved in 8 Type 2 inflammatory diseases and is the #1 prescribed biologic among dermatologists, pulmonologists, allergists, and ENT specialists
- Strong uptake in newer indications: COPD, chronic spontaneous urticaria, and prurigo nodularis
Management emphasized Dupixent's unique safety profile, noting it targets a largely vestigial part of the immune system that drives allergic inflammation without suppressing overall immunity. Unlike JAK inhibitors or OX-40 approaches, Dupixent doesn't carry black box warnings about infections or risks like Kaposi sarcoma.
The Sanofi collaboration profit share reached $1.5 billion in Q4 alone, up 42% YoY, driven by Dupixent strength and improving collaboration margins.
Other Revenue Growing: Other revenue (profit share/royalties from license agreements plus contract manufacturing) grew 33% to $239 million in Q4. Notably, Ilaris net sales exceeded $1.5 billion in 2025, achieving the top royalty tier of 15% for the first time.
A major inflection is coming: The Sanofi development balance is expected to be fully reimbursed by mid-2026, unlocking Regeneron's full share of global Dupixent and Kevzara profits.
The EYLEA Transition Challenge
The ophthalmology story is more complicated. While EYLEA HD grew 66% to $506 million in U.S. net sales, the total EYLEA franchise (HD + legacy) fell 28% to $1.1 billion.
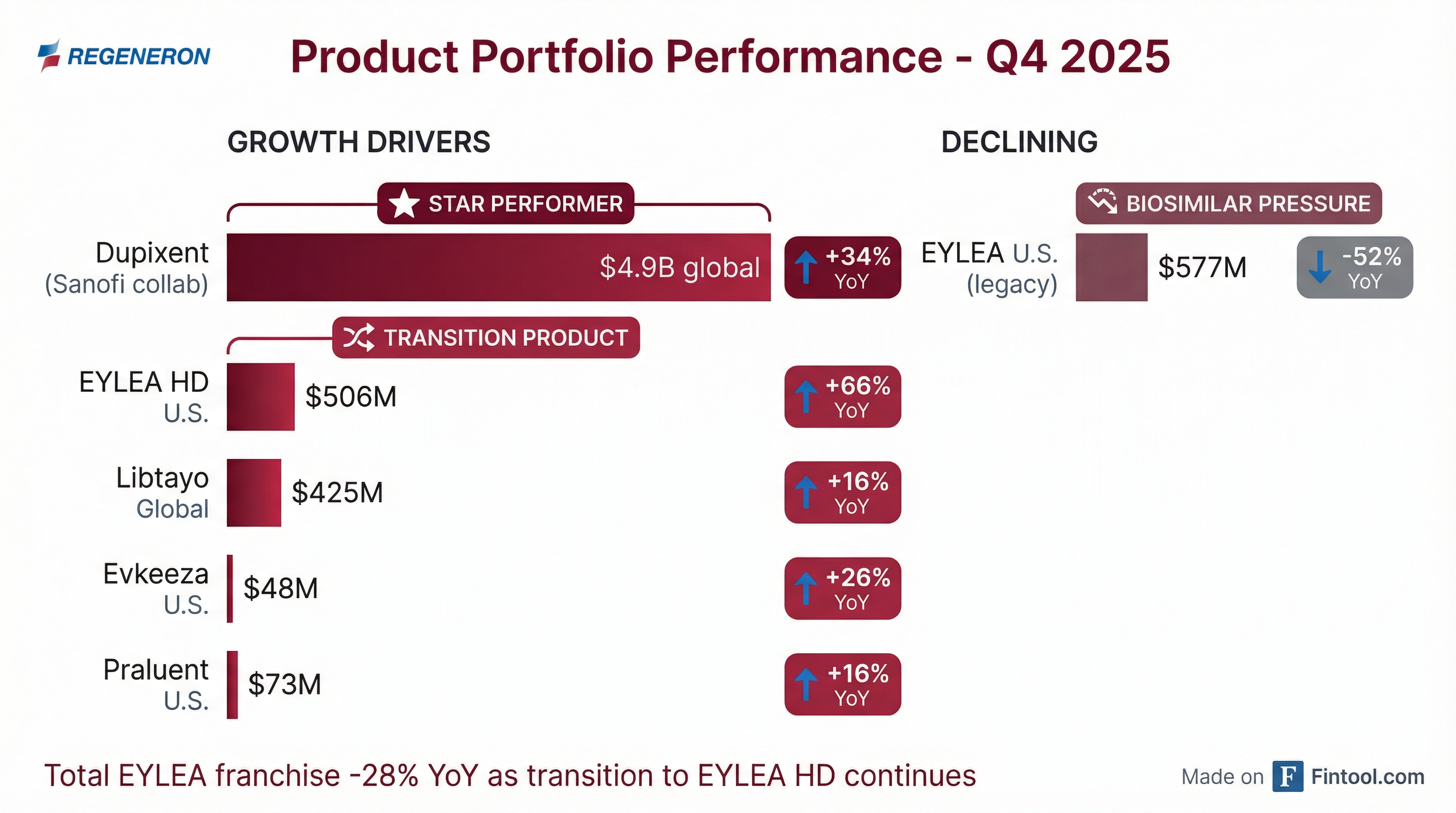
EYLEA HD is gaining share fast. The newer formulation now represents nearly half of total net sales and is seeing strong momentum. Real-world data shows patients switching to EYLEA HD were able to extend treatment duration by almost 4 weeks on average.
The decline in legacy EYLEA accelerated, down 15% sequentially to $577 million due to:
- Continued competitive pressures from biosimilars
- Patient migration to compounded bevacizumab due to affordability constraints
- Transition of patients to EYLEA HD
Q1 2026 Outlook: Management flagged two headwinds for Q1: typical patient reauthorization seasonality and ~$30 million in elevated wholesaler inventory at year-end that will need to be absorbed. They expect high single-digit sequential demand growth for EYLEA HD and double-digit declines for legacy EYLEA in Q1.
Management secured important wins for EYLEA HD during the quarter:
- FDA approved treatment for RVO with monthly dosing
- FDA approved monthly dosing flexibility across all indications
- Pre-filled syringe FDA decision expected in late April 2026
The company also extended its Good Days matching program through year-end 2026, committing up to $200 million to help patients afford their medicines.
Libtayo Momentum Continues
Libtayo delivered $425 million in Q4 2025 global net sales, up 13% year-over-year at constant currency. Full year 2025 sales reached $1.45 billion. U.S. net sales grew 14% YoY to $285 million.
Key Libtayo developments:
- #2 most prescribed immunotherapy for first-line advanced NSCLC in the U.S., with new patient share greater than Opdivo, Tecentriq, and Imfinzi combined
- Market-leading immunotherapy for advanced non-melanoma skin cancers
- Strong launch in adjuvant CSCC following Q4 FDA/EC approvals, now added to NCCN guidelines as the only Category One preferred immunotherapy
- 5-year survival data recently reported, further supporting clinical positioning
Linvoseltamab Early Launch Progress
Regeneron's BCMA x CD3 bispecific linvoseltamab for relapsed/refractory multiple myeloma is showing strong early traction. Management called out its differentiated profile:
- Nearly double the complete response rate vs. other BCMA x CD3 bispecifics at similar follow-up
- Lower rates of cytokine release syndrome
- Shorter hospitalization requirements
- More convenient dosing intervals
Physicians are appreciating the less burdensome profile, with management expecting adoption to build steadily. The larger commercial opportunities lie in earlier lines of therapy, where registrational studies are now underway.
How Did the Stock React?
Down 2.5% in aftermarket trading despite the double beat.
The stock closed at $749.44 on January 29th and traded down to $731 in after-hours. The negative reaction likely reflects:
- EYLEA franchise erosion — The 28% decline signals biosimilar pressure is intensifying faster than EYLEA HD adoption
- Margin compression — Non-GAAP gross margin on net product sales fell to 85% from 86% YoY
- Higher tax rate — Non-GAAP ETR of 17.1% vs 9.9% last year crimped EPS growth
The stock is down approximately 30% from its 52-week high of $812 reached in December 2025, trading near its 50-day moving average of $754.
What Did Management Guide?
Management provided full year 2026 financial guidance:
Key callout: Non-GAAP gross margin guidance of 83-84% is below 2025's 85%, reflecting changing product mix and investments to expand bulk manufacturing capacity and fill-finish capabilities.
R&D increases are driven by expanding late-stage programs in oncology/hematology, Factor XI antibodies, obesity, and advancing several new molecules into clinic across ophthalmology and immunology.
SG&A investments support ongoing launches of Libtayo in adjuvant CSCC and linvoseltamab in late-line myeloma, plus potential launches including cemdisiran in gMG.
Sanofi Development Balance Update: The balance owed to Sanofi has fallen to just ~$600 million at year-end and is expected to be fully reimbursed by mid-2026. Once cleared, Regeneron will receive its full share of global Dupixent and Kevzara profits without the reimbursement drag.
Key Late-Stage Pipeline Programs
Regeneron highlighted major late-stage opportunities that could drive future growth. Management outlined ambitious 2026 targets: at least 4 FDA approvals (including 3 new molecular entities), 18 additional Phase 3 studies initiating with cumulative enrollment of ~35,000 patients, and clinical development start for at least 3 first-in-class antibodies.
Linvoseltamab: Potential Myeloma Paradigm Shift
Linvoseltamab's data continues to stand out. On the call, management highlighted extraordinary MRD (minimal residual disease) negativity rates:
- Newly diagnosed myeloma: 100% MRD negativity (9/9 evaluable patients at planned Phase 3 dose)
- High-risk smoldering myeloma: 100% MRD negativity (12/12 evaluable patients) vs. daratumumab's <10% complete response
- Second-line light chain amyloidosis: Normalized abnormal light chains in ~2 weeks vs. ~5 months for daratumumab quad combo
Management called this a potential transformation of the myeloma treatment paradigm, moving away from complex multi-drug combinations toward linvoseltamab monotherapy or simple combinations.
Fianlimab + Libtayo (LAG-3 + PD-1): The pivotal study in 1L metastatic melanoma is on track for H1 2026. Management noted the study is not using PD-L1 expression as an inclusion/exclusion criteria, representing a true first-line advanced melanoma population. The study is powered for both PFS (the primary endpoint) and overall survival benefit.
Cemdisiran (C5 siRNA) in gMG: Met primary and all key secondary endpoints with 2.3-point placebo-adjusted improvement in MG-ADL — the highest observed among C5 inhibitors. Convenient every-3-month subcutaneous dosing vs. competitors' more frequent IV/SC regimens.
Factor XI program: Management emphasized differentiation from small molecule approaches like Bayer's asundexian. Unlike small molecules that inhibit multiple proteases and have off-target effects, Regeneron's antibodies offer superior specificity, potentially better anticoagulation, and critically, less bleeding risk — all with convenient once-monthly (or post-procedure) dosing.
Geographic Atrophy: The Phase 3 study design includes a prospective secondary endpoint for 15-letter visual acuity loss at years 1 and 2, unlike incumbent therapies that only evaluated this post-hoc. Management highlighted the systemic approach (avoiding intravitreal injections) could address the safety concerns that have limited adoption of approved GA therapies, which can paradoxically cause blindness through retinal vasculitis.
Q&A Highlights: What Management Revealed
The "Souped-Up Dupixent" Follow-On
One of the most interesting revelations: Regeneron is developing an enhanced version of Dupixent internally dubbed "supi-dupi." CSO George Yancopoulos explained that they've been generating millions of antibody variants over the years and believe they have one that may be longer-acting with other advantages. This candidate will enter the clinic alongside other long-acting IL-4 receptor, IL-13, and IL-4/13 bispecific antibodies expected in clinic by 2027.
Importantly, while not formally in the Sanofi alliance, if this molecule enters full development, it would be done with Sanofi.
Obesity Strategy: The PCSK9 "Trojan Horse"
Management was candid that they don't intend to compete head-on for weight loss. Instead, they're pursuing a differentiated combination strategy — co-formulating their GLP-1/GIP agonist (olorelapide) with Praluent (PCSK9 inhibitor) in a single weekly injection.
The pitch: Imagine a GLP-1 that also lowers LDL cholesterol by 50-60%. Current GLPs don't meaningfully impact cholesterol, yet 50%+ of obese patients also suffer from hyperlipidemia with elevated cardiovascular risk.
CEO Len Schleifer called it a potential "Trojan horse" for cardiovascular health:
"They just want to lose their weight, and they're not even going to realize that they're going to be helping their heart, they're going to be decreasing the overall rate of cardiovascular disease and death in this country."
The clinical program for this combination is expected to begin later in 2026.
Dupixent IP: Potential Extension to 2040+
When asked about Sanofi's commentary on potentially extending Dupixent's runway well beyond 2040, management deferred to Sanofi's explanation but highlighted the portfolio of follow-on molecules as providing additional long-term optionality.
What's Coming in 2026?
Regeneron laid out an ambitious catalyst calendar for 2026, targeting at least 4 FDA approvals including 3 new molecular entities.
Regulatory Decisions:
- Late April 2026: FDA decision on EYLEA HD pre-filled syringe
- H1 2026: FDA decision on DB-OTO gene therapy for hearing loss (first NME to receive FDA Commissioner's National Priority Voucher)
- H2 2026: FDA and EC decisions on garetosmab for FOP (99% reduction in abnormal bone formation at 56 weeks)
- Late 2026/Early 2027: Potential cemdisiran approval in gMG (NDA submission Q1 2026)
Clinical Readouts:
- H1 2026: Phase 3 results for fianlimab + Libtayo in first-line metastatic melanoma
- H1 2026: Interim analysis for fianlimab + Libtayo in adjuvant melanoma
- H1 2026: Phase 2 data in advanced NSCLC for LAG-3 + PD-1 combination
- H2 2026: Interim data from cemdisiran Phase 3 study in geographic atrophy
- Late 2026/Early 2027: Phase 3 results for cemdisiran + pozelimab in PNH
New Clinical Starts:
- Coming months: Long-acting IL-13 antibody entering clinic for atopic dermatitis
- H1 2026: First-in-class immunology antibody from RGC genetics for lupus, Sjögren's, and primary biliary cholangitis
- Later 2026: PCSK9 + GLP-1 combination clinical program
- 2026: Clinical development starts for long-acting glaucoma antibody and thyroid eye disease/Graves' disease antibody
Allergy Pipeline Progress: Initial Phase 3 data from cat and birch allergy programs demonstrated that allergen-specific monoclonal antibody cocktails can meaningfully address ocular endpoints, adding to earlier data showing reductions in nasal, respiratory, and skin endpoints.
- Confirmatory Phase 3 for cat allergy initiating in H1 2026
- Confirmatory Phase 3 for birch allergy already underway
- Data to be presented at upcoming Quad AI conference
Key Quotes From Management
Leonard S. Schleifer, M.D., Ph.D., CEO on 2026 priorities:
"Looking ahead to the next 12 months, Regeneron has several key objectives... We anticipate at least four FDA approvals, including three for new molecular entities across three distinct modalities... 2026 will be an important clinical development execution year as we anticipate initiating 18 additional Phase 3 studies, with cumulative target enrollment of approximately 35,000 patients over multiple years, setting the foundation for Regeneron's next wave of potential blockbuster products."
George Yancopoulos, M.D., Ph.D., CSO on Dupixent's unique profile:
"What people don't appreciate and understand is when we discovered Dupixent and understood this whole pathway, we realized that this was a specific pathway that was driving only allergic disease, and it was a vestigial, largely vestigial part of the immune system that was no longer required to fight most pathogens. That's why, unlike most other immunomodulators, it doesn't suppress overall immunity."
George Yancopoulos on pipeline philosophy:
"This is perhaps one of the first, if not the first company, that bet its entire future on the power of genetics, first mouse and now human genetics. We make our choices based on the most powerful available data and technology that can guide decision-making, which is large-scale human genetics, which allows us to use AI in ways that other people can't."
CFO Chris Fenimore on financial position:
"Regeneron's strong performance in 2025 positions us well to continue investing in our differentiated pipeline, to deliver significant advances for patients and deploying capital to drive long-term value for shareholders."
Capital Allocation Update
Share Repurchases: Regeneron repurchased $3.4 billion in shares during 2025 and returned $3.8 billion total to shareholders. As of December 31, 2025, $1.5 billion remained authorized for repurchases. Management continues to be "opportunistic buyers" of shares.
Dividend: The board authorized a quarterly dividend of $0.94 per share ($3.76 annualized), payable in March 2026. Management views the dividend as a way to expand the pool of potential Regeneron shareholders to include dividend-mandated funds, while share repurchases remain the primary capital return mechanism.
Free Cash Flow: Regeneron generated $4.1 billion in free cash flow in 2025.
Balance Sheet: Cash and marketable securities, less debt, totaled $16.2 billion at quarter end.
The Bottom Line
Regeneron delivered a clean beat on expectations, but the market is pricing in accelerating EYLEA erosion. The bull case centers on Dupixent's continued dominance, pipeline optionality in obesity and oncology (with the differentiated PCSK9 + GLP-1 combination), and substantial firepower for M&A. The bear case: gross margin compression, a decelerating EYLEA franchise, and execution risk on an ambitious 2026 catalyst calendar that includes 4 FDA approvals and 18 new Phase 3 studies.
With $16.2 billion in net cash and a P/E around 17x forward earnings, Regeneron trades at a discount to large-cap biotech peers. The stock's reaction suggests investors want to see EYLEA HD adoption accelerate and the linvoseltamab/fianlimab pipeline programs deliver before giving credit. The H1 2026 fianlimab melanoma readout will be a key test of management execution.